CDS chemistry
Chlorine dioxide is an inorganic compound consisting of the element chlorine (Cl) and oxygen (O). Its chemical formula is ClO2. It is a yellow-greenish-gas. It does not occur naturally in the environment. In the reaction process between sodium chlorite and hydrochloric acid, chlorine dioxide, common salt and water are formed.
5 NaClO2 + 4 HCl → 4ClO2 + 5 NaCl + 2 H2O
There are other forms of production but they are not relevant in this space.
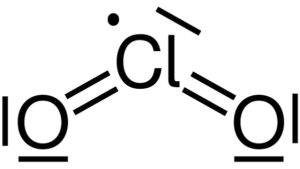
Lewis structure of chlorine dioxide ClO2. Yikrazuul [Public domain]. Source: Wikimedia Commons.
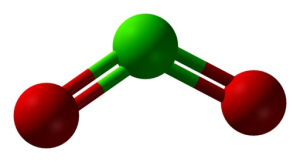
Structure of ClO2 in three dimensions. Green=chlorine; red=oxygen. Ben Mills and Jynto [Public domain]. Source: Wikimedia Commons.
It is important to differentiate between the properties of chlorine dioxide as a gas and as an aqueous solution.
Properties as a Gas:
Link: https://gestis-database.dguv.de/data?name=001640
– Physical state: Gas, yellow-green to yellow-red in color.
– Molecular weight: 67.45 g/mol.
– Melting point: -59 ºC.
– Boiling point: 11 ºC.
– ORP (oxidation-reduction potential): 0.94V.
– Density: 1.642 g/cm3 @ Temp: 0 °C, Gas = 2.33 (relative density to air, air=1).
– Vapor pressure: 140 kPa at 20 °C.
– Critical temperature: 192 °C.
– Chlorine dioxide has a molecular weight of 67.46 and a standard oxidation state of +4 for the Cl atoms.
– It has a boiling point of 11 ºC, a melting point of -59 ºC, a density of 1.64 g/mL (liquid) at 0 ºC, a water solubility of 3.0 g/L at 25 ºC, and a pKa value of 3.0. It is highly soluble in water and does not hydrolyze.
– It has a chlorine-like odor and is toxic if inhaled in large amounts over an extended period.
– It is soluble in water, very soluble, and does not hydrolyze.
– Concentrated ClO2 vapors are potentially explosive, especially when present in the air at concentrations greater than 10%. They should not be compressed, whether alone or mixed with other gases, due to the risk of explosion from compression or the effect of solar UV light.
– ClO2 as a gas decomposes instantly upon contact with organic materials. It can also react in the presence of mercury (Hg) or carbon monoxide (CO).
– Under the action of ultraviolet light (UV) or ozone, ClO2 converts into chlorine hexoxide (Cl2O6), a highly unstable compound.
Properties of Aqueous Solutions:
Link: https://gestis-database.dguv.de/data?name=531775
– Classified as Food Additive E-926.
– Solubility in water: Concentration: 3 g/l – Partial pressure: 4.6 kPa – Temperature: 25 °C.
– Physical state: Gas dissolved in water, yellow in color (CDS).
– Aqueous solutions of chlorine dioxide are greenish-yellow in water, and their degree indicates their concentration.
– In aqueous solution, chlorine dioxide is highly soluble and does not hydrolyze to form other molecules with the hydrogen of water.
– They are stable when well sealed in brown glass bottles.
– CDS diffuses through plastics due to its small size of approximately 140 picometers.
– It is recommended to keep the CDS concentrate cold, well sealed, and protected from sunlight.
– In the presence of light, it slowly decomposes, forming hydrochloric acid (HCl) and chloric acid (HClO3), lowering the pH of the solution.
– In alkaline solutions, ClO2 decomposes into chlorite ions (ClO2-) and in very alkaline solutions, above pH 10, into chlorate (ClO3-).
– In acidic solutions, chlorous acid (HClO2) is formed, which then decomposes into hydrochloric acid (HCl) and chloric acid (HClO3).
– The ultraviolet absorption spectrum of ClO2 solutions shows a broad band with a peak at 360 nm and a molar extinction coefficient of ~1250 M-1 cm-1.
– It is important to differentiate between the mixture of sodium chlorite with an acid and the gas dissolved by itself, as they behave differently. The mixture creates a continuous oscillating reaction, while the gas dissolved in water is stable and known as CDS. The mixture has a redox potential of +5, while the gas dissolved in water has a redox potential of +4 and does not react with HCl.
– It is used to purify water and make it safe for drinking due to its effectiveness against viruses, bacteria, and fungi.
Medical Applications:
– Aqueous solutions of ClO2 have been used to treat oral candidiasis (mouth infection). Candidiasis is an infection caused by the fungus Candida albicans. Chlorine dioxide eliminates the fungus from the mouth and significantly improves the appearance of oral tissues without side effects.
– ClO2 solutions applied to surgical operation wounds can decrease or suppress adhesion formation without affecting the healing process, with the additional advantage of their antiseptic properties.
– It has been used and approved for the disinfection of blood donation bags against viral contamination in 1993 (Alcide).
– It has been successfully used against the Sars-cov-2 coronavirus following the law approved for this purpose in Bolivia and many other countries.
– Due to evidence of its clinical efficacy, there are many ongoing medical researches regarding its many applications.
– Absorption from an aqueous ClO2 solution occurs rapidly within 7-15 minutes. The absorbed chemical agent is likely the gas that is absorbed thru the stomach wall under the rules of the 2nd law of Fick of gas diffusion. It does not react with the HCL acid of the stomach.
